Núria Oriols
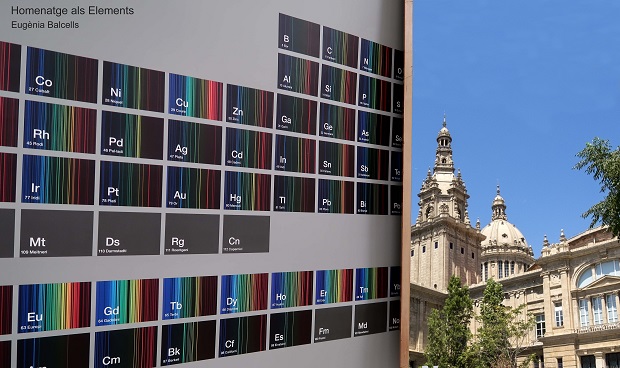
The year 2019 was declared International Year of the Periodic Table by UNESCO. It commemorated the 150th anniversary of a system to organize and classify the 63 chemical elements that were then known, designed by the Russian chemist Dmitri I. Mendeleev. He based it on atomic mass, and left gaps in which to place new elements that had not yet been discovered, even outlining their properties – an infographic paradigm that does not cease to astonish us.
One hundred and eighteen elements are now known, of which only 92 can be found in nature and only 80 are stable. The rest are radioactive, that is, they disintegrate and are transformed into other elements. A small group of these natural and stable elements are recurrently found in works of art. The periodic table, often described as a cultural icon, is also a catalogue of the components from which the materials that make up works of art can be generated.
Now, as we leave 2019 behind, during which the periodic table was in the news everywhere and was associated with countless fields and disciplines, we could not let the opportunity pass to mention the role of chemistry in art. For this reason we propose, here, a visit to the museum’s rooms, following a very unusual guide: chemical elements.
There is one particular element in the periodic table, carbon, that combined in a majority of cases with hydrogen and oxygen and with a minority of other ones like nitrogen, phosphorus or sulphur, and also sodium and potassium, generates a whole host of compounds: those of organic chemistry. All these elements, to a greater or lesser extent, are found in the polychromed paintings and sculptures in all the museum’s collections. They got there via substances like linseed oil or egg white, which act as agglutinants for pigments; resins too, used for varnishing; or substances used as adhesives, such as casein or animal glues.
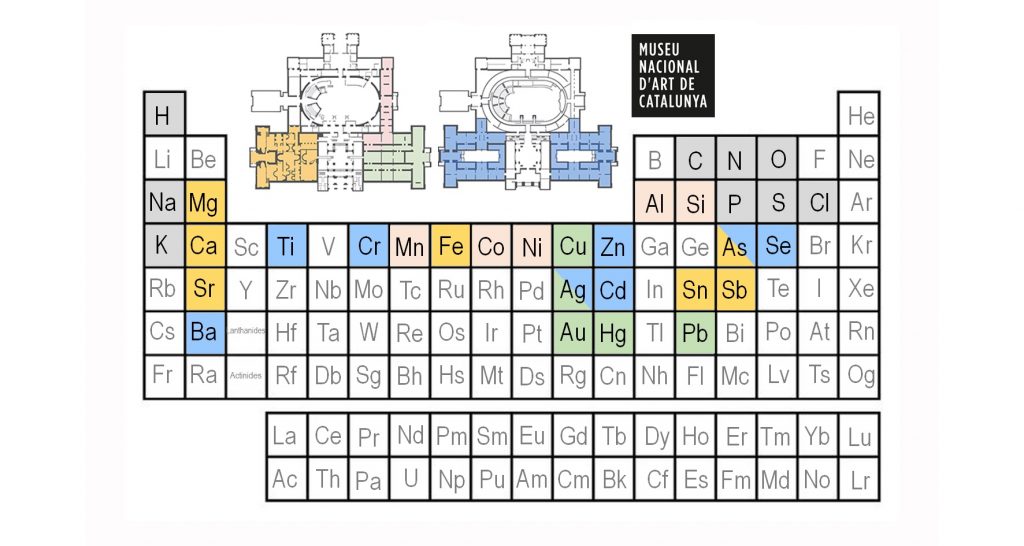
The visit we propose, on the other hand, focuses on transition metals such as iron, copper, cobalt and chrome, which form very brightly coloured inorganic compounds. This is why they are used as pigments. Occasionally, these transition metals also provide additional information about the work’s original date, the reason why the virtual visit suggested here follows a chronological order. Let’s get going!
Romanesque art rooms
When we open the door, we find ourselves standing before the magnificent mural paintings removed and transferred from the apse of Sant Pere of La Seu d’Urgell (12th C). Their colours revolve around earthy hues, blues and greens. Our attention is drawn to the presence of different shades of blue: a deep hue, that of natural ultramarine, or lapis lazuli, in the cloak of the Virgin Mary; and in the background of the mandorla one sees the application of a lighter shade, sky blue, supplied by the mineral called aerinite.
Iron in ochres and blues
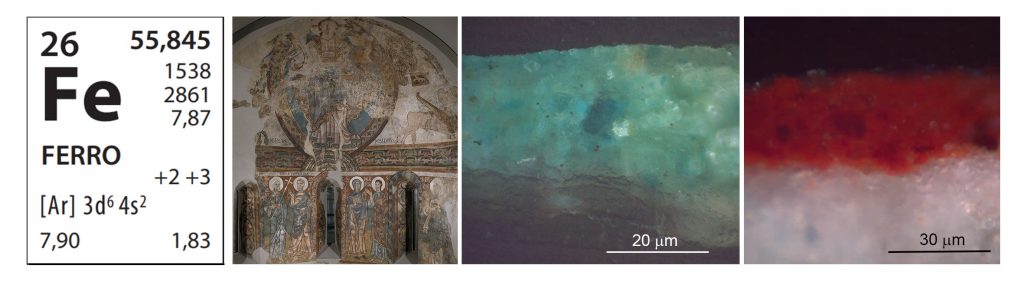
Iron (Fe) – one of the most abundant elements on Earth and among the oldest used by man – appears in this apse. We can quickly associate the colour of the red and yellow ochres that we see in it with the colour of rusty iron. This is because the ochre pigments applied in these hues by the master painters of the eleventh and twelfth centuries contain iron oxide (III), whether haematites, red, or goethite, yellow. Iron is undoubtedly one of the elements most extensively present in the earthy polychromy of all of the Romanesque mural paintings exhibited in the museum.
What is not so obvious, perhaps, is that iron is also found in the blue areas, where there is aerinite. In the chemical composition of this mineral, determined in 2004 through synchrotron radiation X-ray powder diffraction, iron appears in different states of oxidization. In aerinite some cations of iron are as oxidized as in haematites and, therefore, they are iron (III); but others are in a lesser state of oxidization, iron (II). The presence of iron in different states of oxidization might explain the blue colour of aerinite, so characteristic of the Romanesque paintings created on either side of the Pyrenees. It is precisely in this geographical area where we find important deposits of this mineral. Notwithstanding that, aerinite has been identified, for example, in the paintings in San Martín de Elines, in Cantabria, or even in Piedmont, in Italy, in the polychromy on the walls of the Abbazia della Novalesa, probably because this pigment travelled along with other materials from the workshops of Romanesque painters, who were itinerant.
Apse of Sant Pere of La Seu d’Urgell, second cuarter of 12th. Detail of the cloak of the Virgin Mary and the background of the mandorla.
Indeed, the sky-blue colour of this mineral determined the name given to it by Arnold von Lasaulx, director of the Museum of Mineralogy at the University of Breslau, around the year 1876, when analysing some samples of blue stone from Estopanyà (Huesca) labelled as «vivianite from Spain». Upon determining that it was a mineral whose composition was completely different to vivianite (which contains phosphorus together with iron), he used the Greek root aer-, referring to the sky or the atmosphere, to name the new mineralogical specimen.
There is another pigment that contains iron in two states of oxidization and which is also blue. It is ferric ferrocyanide or «Prussian blue». The colour in this compound is explained by the transfer of electrons from one iron (II) atom to an adjacent iron (III) atom. Nevertheless, if the blue of a Romanesque Virgin’s cloak had been painted completely with this pigment, we would have to suspect that it was a forgery or a retouching. This chemical compound was unknown in the Middle Ages! This colour was a chance discovery, totally unforeseen, by the German colour manufacturer Johann Jacob Diesbach, in about 1706, when he was looking for a red colorant… but that’s another story.
Other elements present in the Romanesque rooms
Calcium (Ca), accompanied by magnesium (Mg) and strontium (Sr), three elements aligned in the second column of the periodic table, are habitual in Romanesque works, found chiefly in calcium carbonate mortars and in plaster preparations. Tin (Sn) is also a common element in the altar frontals of the eleventh and twelfth centuries, applied in the form of a metal sheet covered with yellow varnishes, imitating gold. And finally, in these rooms we have the more minor presence of an element that has been identified in objects decorated with enamels from Limoges: antimony (Sb). On another occasion we will explain the fate of the Eucharistic Dove, exhibited in room 16, which contains it.
Gothic art rooms
Gold and gilding
When we enter the Gothic rooms, we are surrounded by altarpieces, a new kind of work, and a clearly different set of colours in which gold takes pride of place. Retro tabula or retables appeared, probably as a consequence of the need for a greater display of iconography behind medieval altars, to complement the frontals. These works of polychromed wood were made by different workshops, based on the promoters’ commission, sometimes formalized in a contract, in which requirements appeared with respect to the quality of the materials, and especially those used in gilding, the unequivocal symbol of wealth, synonymous with prestige, and a vehicle for ostentation.
Thus, in these rooms, the presence of the chemical element with atomic number 79, gold (Au), is prolific. It’s Latin name is aurum, which means «shining dawn», and its alchemical symbol is a radiant solar disk, evoking divinity, thus exalting its properties, in their colour and brilliance, and its chemical integrity with the passage of time. Other heavy metals present in Gothic works are mercury (Hg), with atomic number 80, and lead (Pb), atomic number 82. The latter is very abundant due to the constant and extensive use made of the lead white pigment, although we can also find it in the composition of red and yellow pigments. But let’s take a quick break from our visit and talk about the element copper.
Copper in blues and greens
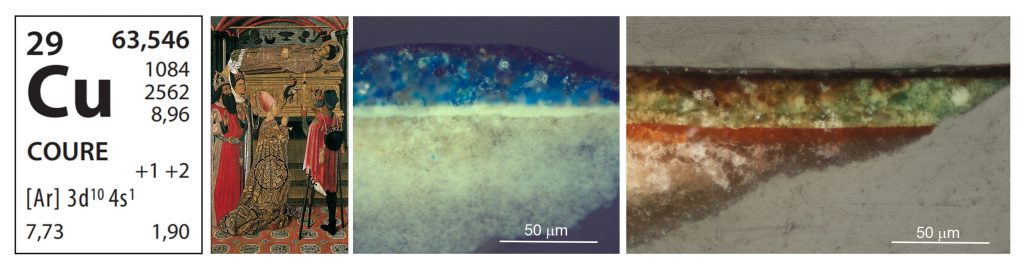
Copper (Cu) is found above all in the blue and green colours in Gothic works of art, introduced through pigments of both natural and synthetic origin. The classical texts mention the greenish or greenish-blue products of corrosion so common on the surfaces of metallic copper and its alloys, referring to it with the term aerugo. Ever since then, the great chemical reactivity of this metal has historically been taken advantage of for synthesizing pigments. In medieval painting treatises, we see different recipes for obtaining green pigments, causing the corrosion of metallic copper with acidic vinegar vapours or with urine. Heterogeneous mixtures of acetates and copper chlorides, in different shades of green, were obtained this way, depending on which additives were used, such as honey or common salt.
The copper (II) cation of copper acetate, a compound called verdigris, can react very easily with the organic acids contained in resins and oils, forming salts of a dazzling and translucent green colour. This property was quickly taken advantage of to give rise to a series of glazed artistic materials, or colres (yellow glazes), that historically have been identified with the term «copper resinates».
Alterations produced by the passage of time
The different green hues of copper were used especially during the Gothic period. As was the azurite pigment, a basic copper carbonate, blue in colour. The pigments and the agglutinants that master painters applied 500 years ago are chemical compounds that react with one another, with other substances around them, and which are affected by the light and the atmospheric conditions. These inevitable reactions and the transformations that take place are responsible for the natural ageing of artistic objects and the formation of alteration products. When the pigments used contain particularly reactive cations, as in the case of copper, quite important changes in the aesthetic perception of works may take place. It is difficult to halt the reactivity of copper at just the right point, and the passage of time eventually causes a notable darkening of the colours that contain it.
Let us now pass, on our visit, in front of the panels exhibited in room 26, painted by the Vergós family group, from the altarpiece of Saint Stephen from Granollers (1492/1494-1500). The colours we observe in it do not always correspond with what the painter intended. There are iconographical elements that are now a different colour to the original ones. For example, the curtains in The Birth of Saint Stephen, apparently black, were painted with copper green. And the tiles, in consonance with the ceramics of the period, had blue decorations and not the pitch-black lines we now see. The azurite, in fact, has remained unaltered and intensely blue within the polychromy because the darkening, in this case, is chiefly superficial, conditioned in part by the morphology and particle size of this pigment. Also polychromed with azurite was the stocking of the figure on the right of the panel Princess Eudoxia before the Tomb of Saint Stephen, now completely black. In order to restore the paint losses in this area, in the past a restorer, ignorant of the original colours, used a carbon black pigment; this has been revealed by recent hyperspectral imaging analysis, which makes it possible to locate, or «map», the different chemical compounds in the work.
In part two we shall continue the visit through the Renaissance and Baroque and Modern Art rooms.
Birth of Saint Stephen and Princess Eudoxia before the Tomb of Saint Stephen. Both compartments from the altarpiece of Saint Stephen from Granollers, Vergós Group, 1495-1500.
Related links
La tabla periódica en el arte (y en las redes sociales) La cultura social. Nacho Granero (04/12/2019)
The magnificence of the «fashion» and the brightness of the colours of the Gothic I Museu Nacional d’Art de Catalunya. Núria Prat (19/02/2015)
The magnificence of the «fashion» and the brightness of the colours of the Gothic II Museu Nacional d’Art de Catalunya. Núria Prat (26/02/2015)
Restauració i Conservació Preventiva












One comment
Thanks for this info!